

Patients with implantable drug delivery systems, e.g.History of an allergic reaction or previous intolerance to transcutaneous electronic nerve stimulation.Use of an investigational agent for pain control concurrently or within the past 30 days.Any of the following: pregnant women, nursing women, women of childbearing potential or their sexual partners who are unwilling to employ adequate contraception (condoms, diaphragm, birth control pills, injections, intrauterine device, surgical sterilization, subcutaneous implants, abstinence, etc.).Patient understands the study regimen, its requirements, risks, and discomforts, and is able and willing to sign an informed consent form.An average daily pain rating of > 4 out of 10.Pain or symptoms of lower extremity peripheral neuropathy of >3 month's duration attributed to chemotherapy-induced peripheral neuropathy.Lower extremity CIPN neuropathy: Received neurotoxic chemotherapy (including taxanes-such as paclitaxel or docetaxel, or platinum-based compounds such as carboplatin or cis-platinum or oxaliplatin, or vinca alkaloids such as vincristine, vinblastine, or vinorelbine, or proteosome inhibitors such as bortezimib).Men and women, 18 years of age or older with cancer.Condition or diseaseĭevice: Scrambler Therapy Device: Sham Therapy We hope that this study will help us determine if the Scrambler device really helps patients with CIPN.Ĭancer patients with chronic, chemotherapy-related pain of 4 or more (on a 0-10 scale) for at least 3 months may be eligible to join this study. In this study we want to compare the Scrambler Therapy with the sham therapy (the therapy that does not use the electrical signals).
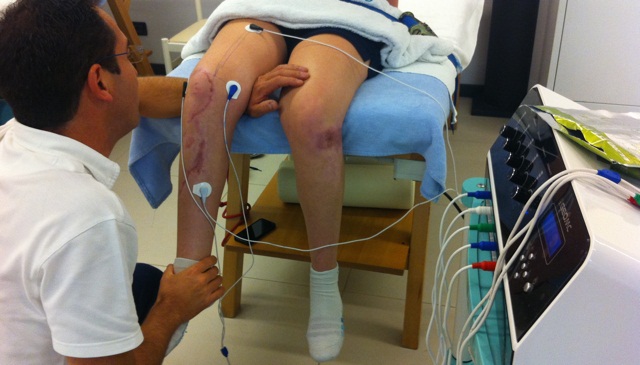
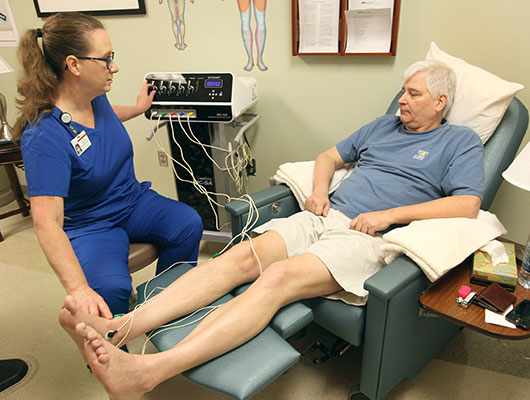
This means that some of the pain relief could be due to placebo effect, or the CIPN pain going away on its own. The electrodes are placed on the body in pairs, and the Scrambler Therapy machine directs electrical signals across the field to simulate non-pain information.īased on other studies, we think that we relieve pain with the Scrambler therapy device, but it has not been tested in a setting such as this one.
#SCRAMBLER THERAPY SKIN#
Scrambler Therapy is a method of pain relief given with common electrocardiography (ECG) skin electrodes. The purpose of this study is to see if Scrambler Therapy with the Calmare MC5-A machine will relieve chemotherapy induced peripheral neuropathy (CIPN).


 0 kommentar(er)
0 kommentar(er)
